European Medical Device Regulation (MDR) is continuously evolving, making it difficult to keep up with all of its requirements. It is important to understand these guidelines to ensure that your medical devices meet the required standards.
Functions of MDR Labeling
In the second part of a three-part webinar series, Ronald Boumans, Senior Consultant for of Regulatory Affairs for Emergo by UL and an expert on MDR labels, discussed the importance of labeling. Proper labels are crucial in order for a device be properly identified under the MDR. The label has five basic functions:
- Identifies the physical product
- Identifies the purpose of the product
- Identifies the manufacturer of the product
- Identifies the product type
- Identifies instructions and product warnings
EUDAMED and Language Access
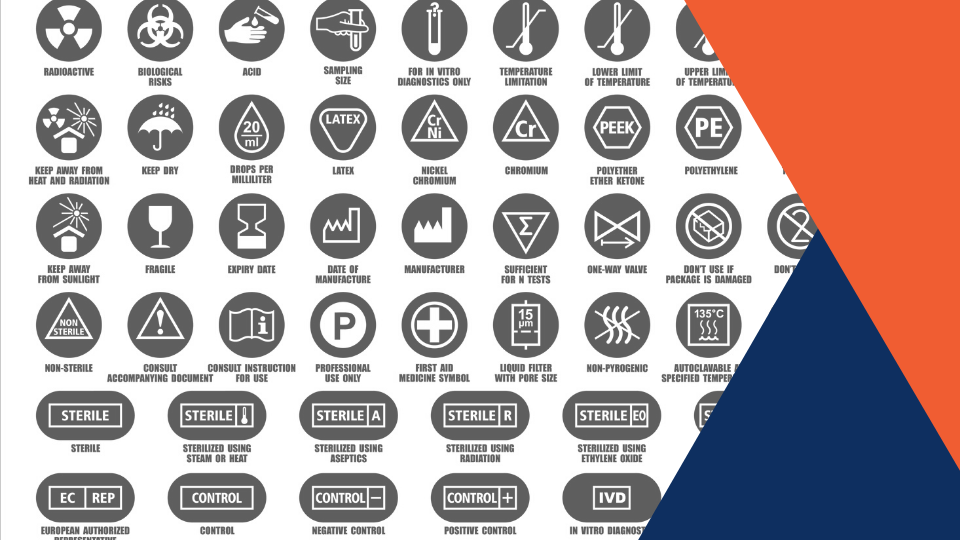
The label enables the manufacturer to speak directly to the person who will use the product. Because the label is a crucial point of communication, it is essential that all text be translated properly. With 24 languages in the E.U., we know how complex your label can get! Symbols, on the other hand, are universal for all 24 official European languages, scoring a big win for your medical device label. Symbols include CE number, expiration date, LOT number, serial number, etc.
It’s crucial that the label clearly identify the product manufacturer. This is necessary in case customers need to contact the manufacturer with questions or concerns. It is not necessary, however, to include a phone number on the label. Products come in all shapes and sizes, and sometimes, it can be tricky to determine how to place a label. Not to worry! If a product is having a hard time fitting a label, the label can be split up into multiple parts.
Labels for MDR can be tedious and difficult to understand. For a deeper dive into MDR labeling, request a recording of "The Perfect Label."
Life Science Language Solutions
At United Language Group we pride ourselves in being industry experts, so you don’t need to take the time to bring us up to speed on regulations, industry-specific guidelines, and the strictest global requirements. We understand scientific language and processes as well as the intricacies of settings in which your translations may be used.
Connect with us today to learn more about ULG's medical device translation services.